Tuberculosis, or TB, an airborne infectious disease, remains one of man’s great adversaries. It is estimated that one third of the world’s population is infected. Although huge strides have been made in reducing the global burden of this disease, a new threat has emerged. Multi and Extensively Drug resistant TB are rewriting the rules.
In 2010, 8.8 million people fell ill with TB and 1.4 million died from it. 95% of these deaths were in the developing world. TB is closely linked with poverty as well as reduced immunity, which is most strikingly seen in the form of increased rates of HIV infection.
Here we examine why drug resistant TB has come into existence, how it can be categorized, what treatment options are available and how the global health community plans to combat this growing threat. We will also look more closely at India, one country particularly bearing the brunt of this emerging challenge.
MDR, XDR, TDR, or XXDR?
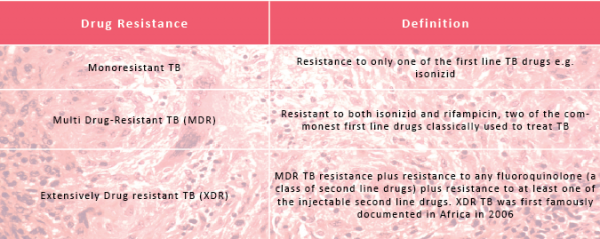
Drug resistant TB can be divided into 3 categories: Monoresistant, Multi Drug-Resistant (MDR) and Extensively Drug Resistant (XDR) TB. There are currently thought to be 650,000 cases of MDR TB worldwide. Of these, 9% are thought to be XDR cases.
There are 27 MDR TB high burden countries as defined by the WHO (World Health Organisation). Those that bear the highest burdens are India and China. Within these regions 73 from every 1000 cases of TB are thought to be multi drug-resistant. At last count, in 2011, 77 countries worldwide had reported at least one case of XDR TB. In the UK, between 2005 and 2010 there were 16 cases of XDR TB.
In India, reports of Totally Drug Resistant (TDR) or Extremely Drug Resistant (XXDR) TB have emerged and present a deadly new threat... the WHO, however, does not yet officially recognise this new resistance category
In India, reports of Totally Drug Resistant (TDR) TB, or Extremely Drug Resistant TB (XXDR) TB have emerged and present a deadly new threat. In January 2012, there were at least 4 cases of possible TDR TB reported by Hinduja Hospital, Mumbai. The WHO, however, does not yet officially recognise this new resistance category because drug susceptibility testing of second line TB drugs is not yet standardized: what might be resistant in one lab in India could be susceptible elsewhere, and hence the reluctance to use the label definitively.
Therapy and Resistance
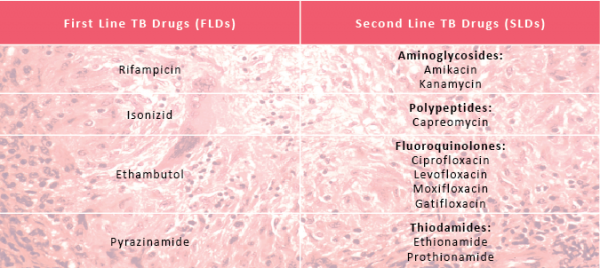
First Line drugs (FDLs) are classically used for treatment of naïve patients where no TB drug resistance has been found. Alternatively, Second Line Drugs (SDLs) are used once drug resistance is suspected or confirmed.
TB physicians are well versed in the uses of first line drugs to treat TB. The efficacy and side effect profile of second line drugs are much less clear, encompassing a wide a range of classes of drugs including aminoglycosides, polypeptides, fluoroquinolones and thiodamides. There are many others that have sometimes been used in desperation, particularly in XDR TB cases.
Inadequate drug treatment can result in spontaneous genetic mutations in Mycobacterium Tuberculosis (MTB) primarily via the selection of resistant strains. Mutations in one gene, enabling resistance to one drug, might also have a knock-on effect, conferring resistance to other drugs.
Hence, drug resistance can occur where TB drugs are of poor quality, where regimes are unsupervised and not properly completed; or where, quite plainly, the wrong drugs have been given.
Drug resistance occurs when TB drugs are of poor quality, or where regimes are unsupervised and not properly completed; or where, quite plainly, the wrong drugs have been given
In India, first line drugs are available without a prescription. There is availability of second line drugs, but stock regularly runs out at both a central and peripheral level, making the supply erratic. As misuse of first line drugs has been the main cause of rising MDR TB in high burden countries, stewardship of second line drugs is a key priority.
Directly Observed Therapy – short term (DOTS) is the cornerstone of treatment of drug sensitive TB and has contributed to significantly to its decline. This is a strategy where, at its heart, patients are observed taking their TB treatment by a trained healthcare worker. DOTS relies on a regular drug supply and simply cannot work if there are no drugs available in the first instance.
Stop TB
The Stop TB Strategy was launched in 2006 by the Stop TB partnership, an international body comprising a variety of partners including the WHO. The Global Plan to Stop TB, also launched in 2006, comprises the nitty gritty of how countries should actually go about doing this, and was updated in 2010 to reflect the growing importance of MDR TB as a very real issue.
A plan for combatting drug resistant TB, therefore, is built into TB frameworks that are already in existence. However, specific policies and strategies aimed at tackling drug resistant TB are now gaining traction. The New Global Framework to support expansion of MDR-TB services and care was launched in 2011.
DOTS services are nation-wide in India, and plans are underway to offer treatment at ‘DOTS-Plus’ sites to those with MDR TB. The DOTS-Plus programme follows international guidelines on the management of MDR TB, pioneered by WHO agencies. The target is to scale-up these services so that they are country-wide by 2012.
The Global Drug Facility, an initiative of the Stop TB Partnership, aims to expand access to, and availability of, high quality TB drugs to facilitate DOTS expansion. It uses innovative strategies to procure TB drugs for countries in need of its services. It is evident that more potent, reliable and affordable drug combinations are needed to treat drug resistant TB.
TB can be difficult to diagnose for any one of a variety of factors whether they be human, lab or operator dependent. As part of the Global Plan to Stop TB, the WHO aims to have at least one functioning TB diagnostic lab per 5 million people world-wide.
Only 16/27 MDR high burden countries have achieved this to date. Very encouragingly, however, all 27 have a lab network capable of testing first line drug susceptibility. India, for example, has improved lab capacity and quality assurance, but limitations remain on the diagnosis and follow up of MDR TB patients. This is currently, however, at the heart of plans to scale-up services.
An affordable, rapid, point-of-care diagnostic tool for TB infection remains the holy grail of TB control. A new tool was rolled out in 2010 by the WHO. The Xpert MTB/Rif test is a NAAT (Nucleic Acid Amplification Testing) for TB case detection and rifampicin resistance, and could revolutionize TB diagnostics.
Efforts are also underway to develop new drugs. In July 2012, results were released from a randomized prospective study conducted in South Africa that could herald a significant breakthrough in the treatment of TB. A combination named PA-824-moxifloxacin-pyrazinamide was found to be very effective for both drug-sensitive and drug-resistant TB, and is potentially a bright hope for the future.

Money Matters
Both human and financial resources are inadequate when it comes to fighting MDR/XDR TB. It is estimated that in 2011, $0.7 billion was needed for the high burden countries alone, but that there was a shortfall of $0.2 billion in funding.
Currently funding for these activities comes from the Global Fund and other international donors such as UNITAID for many MDR high burden countries. In fact the most recent WHO progress report cites the Global Fund as providing over 90% of financial support for combatting TB resistance in 10 of the MDR high burden countries.
It remains a major challenge to stimulate funding from domestic sources, which is rarely achieved. In India, however, the majority of funding is from the Government of India (with World Bank credit) with funds from other international agencies such as DFID, the Global Fund and USAID.
One of the most resounding of the Millennium Development Goals is to eliminate TB as a public health problem by 2050. In the form of resistance, TB has found a new way to perplex and challenge us, yet it is only in the last few years that a roadmap for tackling increasing levels of TB resistance has emerged. This is certainly an ongoing challenge that we cannot afford to ignore.








