It is estimated that vaccines save more than two million lives per year. However, there are still up to 30,000 deaths from potentially vaccine-preventable diseases every day. Furthermore, whilst there have been great successes in reducing vaccine-preventable diseases, a number are making a recurrence, notably, measles, poliomyelitis, pertussis (whooping cough) and tuberculosis. But, for maternal infections such as group B streptococcus and early infant infections such as respiratory syncytial virus infection, there is now hope for the development of successful vaccines, some based on adjuvants which are designed to boost weak immune response.
Vaccines, administered globally for the prevention of illness, act by stimulating the immune system. The protection conferred by a vaccine may be short-lived or long-lived, depending on a number of factors. One of the most important is the period of time during which one is exposed to the infecting agent. A travel vaccine may need only to protect for a short time, yet other illnesses such as influenza pose a constant threat, and so one needs to be protected against this disease continuously via annual vaccination.
For reasons of immune immaturity, immune deficiency or immune senescence, an individual may not respond in an ideal way to a vaccine, and a ‘boost’ to the immune system would be an advantage. For this reason, adjuvants such as aluminium have been developed, and are included in a number of vaccines, for the purpose of optimising the immune response especially in younger and older members of the population, and in those with impairment of their immune systems.
Adjuvants
Aluminium salts are perhaps the best known adjuvants, known to increase immunity even up to a thousand fold2, and have been in use since the 1920s. Licensed newer generation adjuvants such as MF59 and Adjuvant System 04 (AS04) are used in some influenza vaccines and one of the human papillomavirus vaccines respectively. Adjuvant System 03 (AS03), however, has been associated with the onset of narcolepsy, a rare but serious brain disorder that causes a person to suddenly fall asleep at inappropriate times, posing questions about the safety of large-scale adjuvant use.
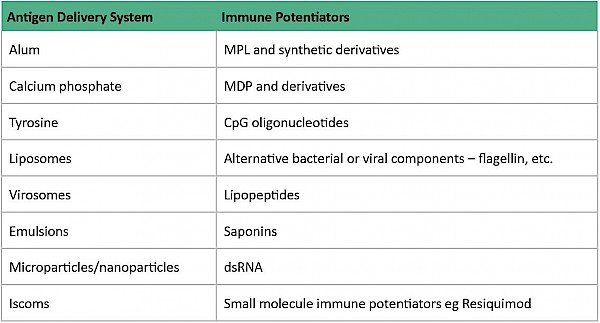
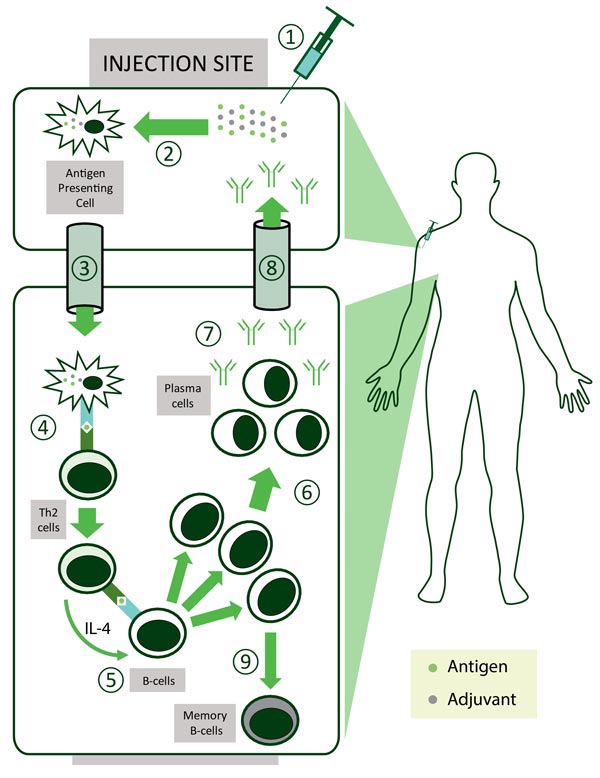
In general, live attenuated vaccines don’t ‘need’ adjuvants, while killed vaccines may need adjuvants and subunit vaccines benefit from adjuvants. ‘Delivery system’ type adjuvants, such as alum, improve antigen uptake by dendritic cells (important cells in the immune system) although their exact mechanism of action remains largely unknown3. It is thought that alum interacts directly with membrane lipids on the surface of dendritic cells, triggering signalling cascades that promote CD4+ T cell activation and humoral immune responses1.
Other types of adjuvants include ‘immune potentiator’ type adjuvants which activate dendritic cells to produce cytokines and stimulatory molecules that are integral for the immune response of the body. The optimal formulation for a subunit vaccine would be that it contains both ‘delivery system’ and ‘immune potentiator’ type adjuvants, thereby producing an effective immune response.
MF59 is an oil-in-water adjuvant composed of squalene and surfactants. In combination with trivalent influenza vaccine, for the prevention of seasonal
influenza, it has been used since 19977.
Can adverse events such as narcolepsy be predicted?
Studied over two influenza seasons in infants and young children, the vaccine was seen to be efficacious against laboratory-confirmed influenza caused by all circulating influenza viral strains during the two study years (86% efficacy rate), with higher efficacy against vaccine-matched strains (89%)9. In contrast, the respective efficacy rates for (non-adjuvanted) trivalent influenza vaccine were 43% and 45%.
So far more than 50 million doses of MF59 adjuvanted trivalent influenza vaccine have been distributed commercially since 1997 and around 100 million doses of pandemic influenza 1 (H1N1) vaccines containing the MF59 adjuvant were distributed in 2009-2011 to all aged groups, including pregnant women, and no safety concerns were revealed9.
The adjuvant AS04 is used in one of the human papillomavirus (HPV) vaccines – it was approved for use in Europe in 2007 and in the USA in 2009. The AS04- adjuvanted HPV vaccine induces a high and sustained immune response against HPV and has an excellent safety profile. By contrast, the adjuvant AS03 is com- posed of α-tocopherol, squalene and polysorbate 80 in an oil-in-water emulsion. However, its use in an influenza A(H1N1) pandemic vaccine was associated with the onset of narcolepsy4 5.
Health Policy Implications
Should concerns about narcolepsy imply that there should be a ‘moratorium’ on all adjuvanted influenza vaccines? Should all adjuvanted vaccines under development undergo extra safety checks to ensure that they are not associated with the onset of narcolepsy? Are the risk-benefit ratios different for public health emergencies such as an influenza pandemic when compared with a routine immunisation programme? Can adverse events such as narcolepsy be predicted? These are the type of questions facing public health experts in the field of vaccine development and delivery.
On rare occasions, adverse events including conditions such as Guillain-Barré syndrome are associated with vaccination, although there is no suggestion that adjuvants are responsible; on other occasions there is proven to be no association between vaccination and a condition – the example par excellence being MMR and the absence of association with autism.
The association between narcolepsy and AS03-adjuvanted pandemic influenza vaccine was not predicted – it was only identified after the widespread administration of the vaccine - and the exact mechanism has not yet been elucidated. It is, in fact, unusual for relatively rare conditions to be detected in even a moderately-sized clinical trial - and most influenza vaccine clinical trials are small, short trials of long-established seasonal influenza vaccines.
Given the long track-record of MF59, a ‘moratorium’ on all adjuvanted influenza vaccines would appear to be unnecessary, and would deny those who benefit from the increased immunogenicity conferred by an adjuvanted influenza vaccine the opportunity to be properly protected. In this case, the preferred approach would be to ensure vigilance and safety monitoring in parallel with the use of the vaccine.
Furthermore, when considering a public health emergency such as an influenza pandemic, the risk-benefit ratio need not be any different from that in a non-emergency situation. This is because pre-pandemic planning and vaccine development is an on-going and continuous process – the vaccines which will be used in the next influenza pandemic are already under intense study, albeit in their pre-pandemic form.
A new era in influenza vaccination has already commenced in the UK
Indeed, a new era in influenza vaccination has already commenced in the UK with the launch of routine paediatric influenza vaccination using an inhaled cold- adapted live (non-adjuvanted) vaccine. Although this is a non-adjuvanted vaccine, some investigators are already exploring the possibility of improving the immunogenicity of such mucosal vaccines with adjuvants8. Current research is also focusing on both the development of adjuvants that can be linked to specific vaccines in order to optimise immune responses, and on delivery mechanisms such as inhaled and transdermal delivery systems, in the hope of overcoming the ability of organisms to evade vaccine immunity.